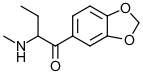
Butylone
Gold Bromide (HAuBr4)
Butylone in gold bromide reagent gives small (15-30 μm), red, and rod-like prisms that grow abundantly but slowly (~ 15-20 mins). The crystals grow abundantly once crystal formation begins. The distinct red crystals are bright under crossed polars and go completely extinct upon rotation of the stage.
Platinic Chloride (H2PtCl6)
Platinum based reagents are the best reagents for butylone, forming the largest and easily characterized crystals. Aqueous and acidic solutions of butylone form tufts of colorless, long rectangular blades with platinic chloride reagent.
The large crystals are easily studied with transmission infrared microspectroscopy and give information-rich spectra.
IR Spectrum - Platinic Chloride (H2PtCl6)
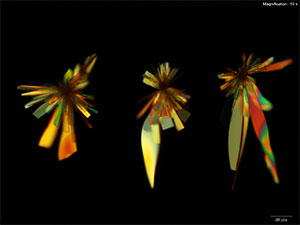
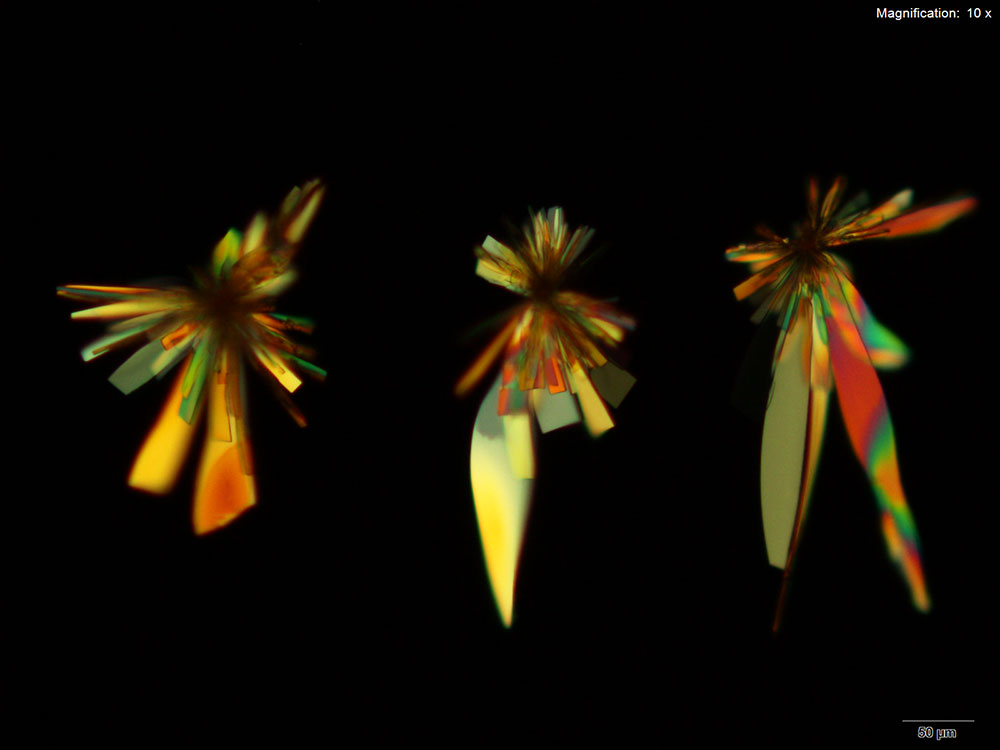
Platinic Bromide (H2PtBr6)
Flat blades and tablets growing in clusters and rosettes are formed with platinic bromide reagent. The long blades have curved sides giving the individual crystals a characteristic leaf like appearance. The crystals grow abundantly within 10 minutes for 5 μg of butylone.