Anodic Porous Alumnia

This is a project that we just started working on. Jennifer Intrieri began our work. She used anodic etching at 20 V or so in a sulfuric acid solution to prepare porous alumina. The aluminum substrate is anodically etched and simultaneously the interface with the solutions oxidizes. A delicate balance between dissolution and oxidation leads to the formation of pores. The pore walls do not dissolve once they have formed because alumina is an insulator and does not easily pass charge either in the form of electrons or ions. Therefore, charge can only be forced through alumina (Al2O3) if the alumina is thin and a high electric field is present. This condition is fulfilled at the bottom of the pore; therefore, the pore bottom continues to etch and the pore propagates. The pores, however, a much present a much longer path to the conducting alumium substrate and not charge flows through them.
Bryan Kelly took over from Jen and advanced the project further. He upgraded the experimental apparatus and performed many investigations of the structure of the films using scanning electron microscopy (SEM). Here he's shown preparing a sample for analysis. The porous alumina is an electrical insulator and, therefore, has to be coated with a AuPd alloy before imaging.


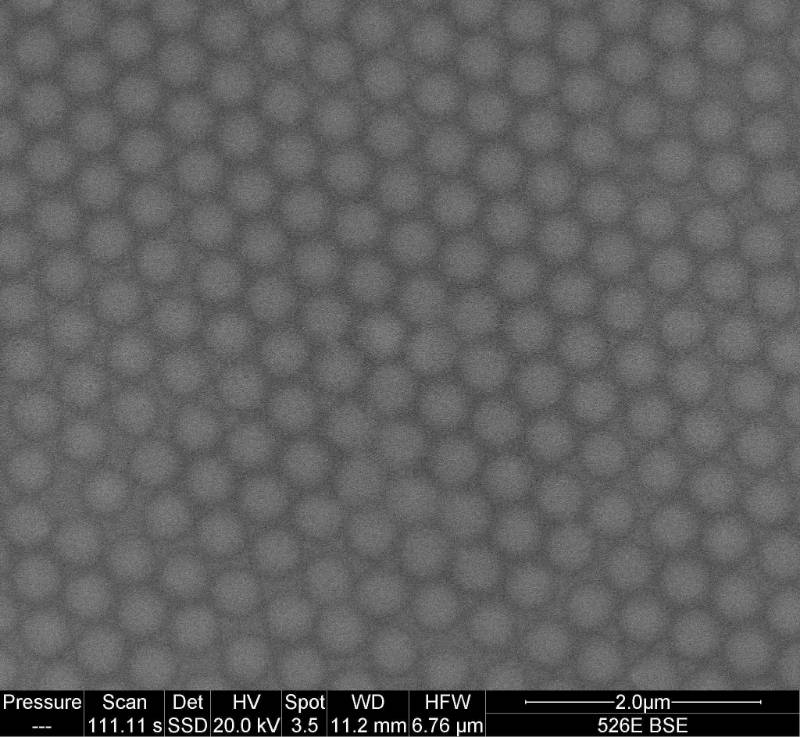
Carolyn Somerville, Jacob Gogola and Nathan Noyes subsequently took over this project and have since perfected the formation of ordered monolayers of polystyrene spheres.
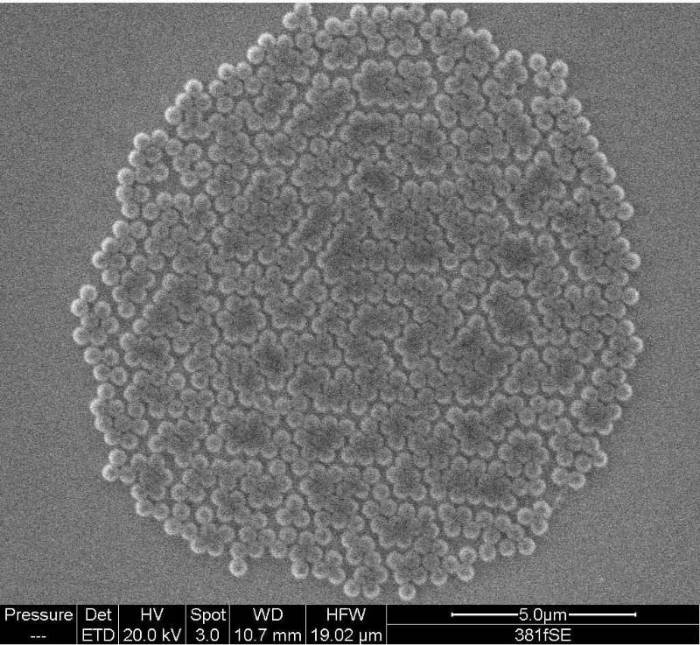
Now Abbie Ganas has taken over, we finally have real TiO2nanotubes as well as other crazy structures such as Al2O3nanobeams.


For further information on related topics, try these sites:
